Oribatid mites (Hornmidd)
The oribatid mites (Acari, Sarcoptiformes) are tiny arachnids. Another meaning of the word “mite” in English is, quite appropriate, “a very small amount“. In some ways the mites resemble spiders, for example do the nymphs and adults have 8 legs, and they molt, which means that they leave their “old skin“, during the development.
The Oribatida are commonly called “moss mites” because they were first found in mosses and it was believed they only lived there. They are also called “beetle mites“ or “armored mites“ (in Norwegian “hornmidd”) because many species have hard exoskeletons, like beetles. They are represented worldwide by more than 11 000 species.
We here present a key to all families of Oribatida known from Norway and a selection of ten Oribatid mite species.
Description
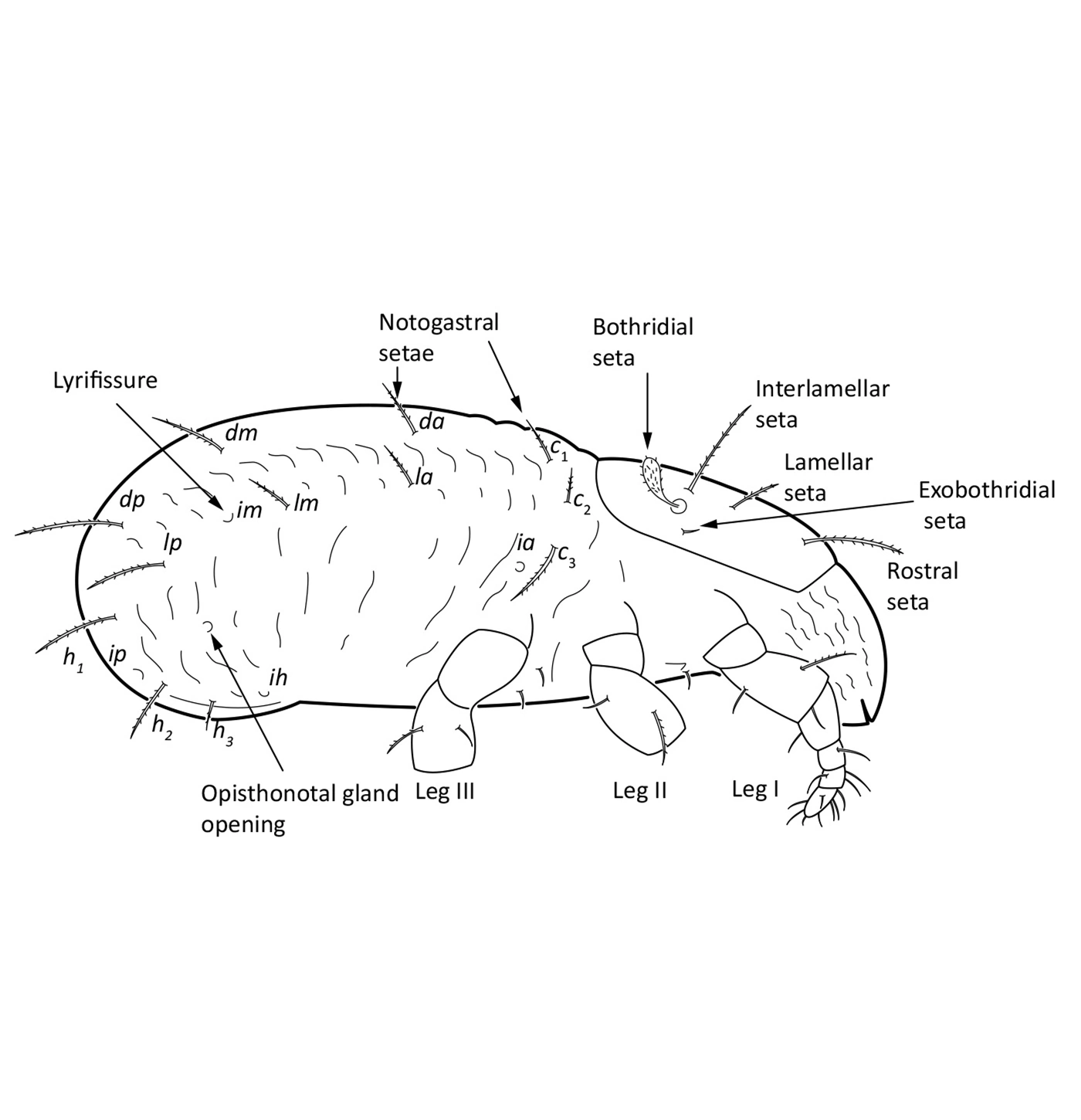
Fig. 1. Hexapod larva.

Fig. 3. Leg with five free segments.
Fig. 5. Dorsal aspect.
Fig. 6. Prolamella.
Fig. 8 Tutorium.
Fig. 10. Shoulder projections.
Fig. 11. Lenticulus.
The body length of adults varies from ca. 150 µm to 2000 µm, but usually is within the range 300–800 µm. Juvenile stages are soft-bodied and light colored. Adults are usually pigmented and their color varies from yellow and orange, through shades of brown. Body shape is usually oval or rounded, sometimes cylindrical or rectangular. The larva is hexapod (i.e. has six legs) (Fig. 1), while the three other juvenile stages (protonymph, deutonymph, tritonymph), and adults are octopod (have eight legs) (Fig. 2). Legs usually consist of five free segments (Fig. 3): coxa, trochanter, femur, genu, tibia, and tarsus. Only in the superfamily Palaeacaroidea, represented in Norway by family Palaeacaridae two femora are present (Fig. 4). Tarsi have 1–3 claws.
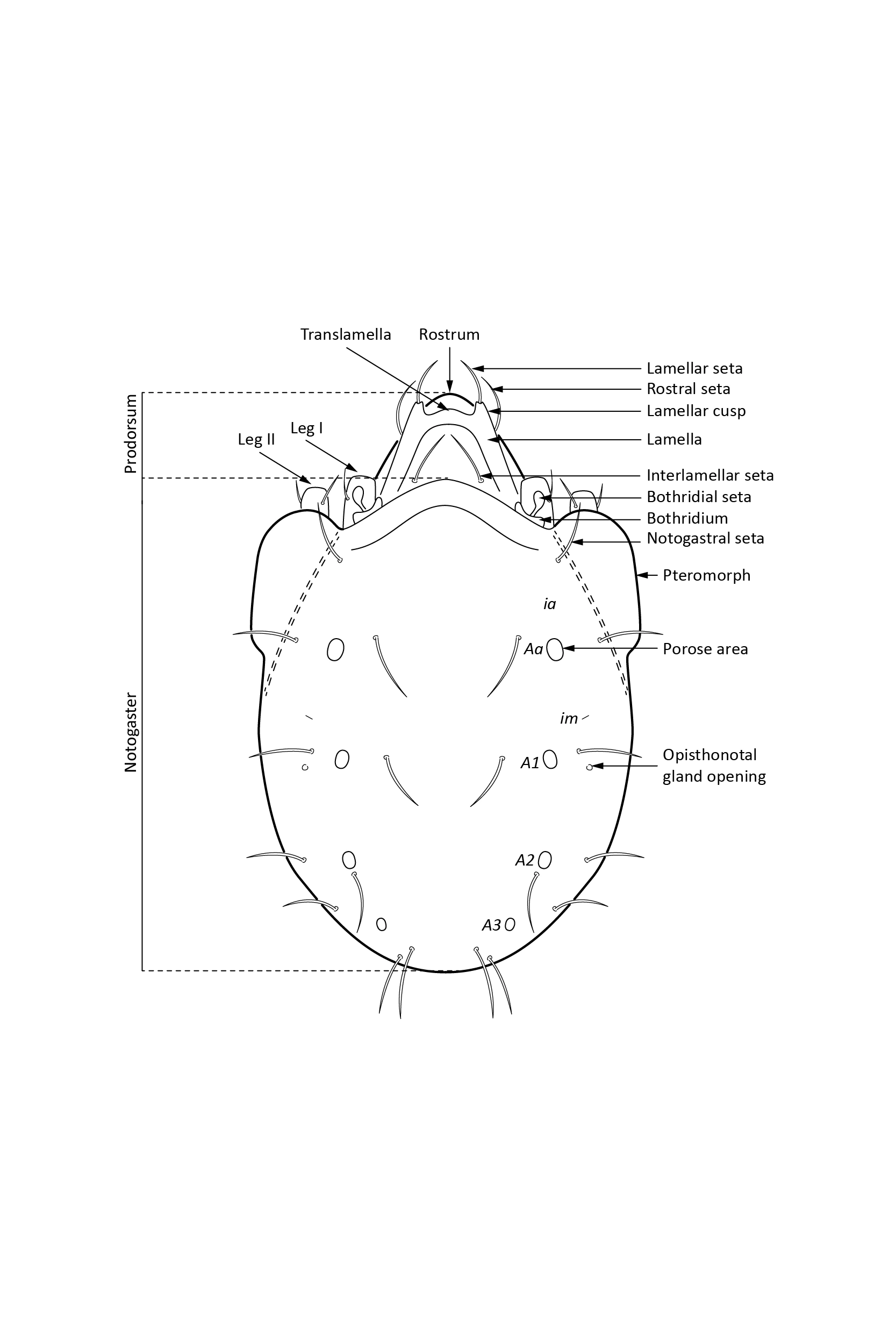
In dorsal aspect (seen from above) two body regions can be distinguished (Fig. 5): prodorsum (frontal, smaller part) and notogaster (main, larger part). Prodorsum has specific setae (i.e. structures resembling hair): a pair of rostral setae, lamellar setae, interlamellar setae, 0–2 pairs of exobothridial setae (seen in Fig. 1), and a pair of bothridial setae, each is usually inserted in a cup-like structure called bothridium. Prodorsum of adults may also have a pair of lamellae, sometimes with a free end, called cusp. Sometimes instead of lamellae, lamellar costulae are present. Translamella is a ridge connecting lamellae. Other ridges that might be present on prodorsum are prolamella (Fig. 6), sublamella (Fig. 7), tutorium (Fig. 8). In Suctobelbidae there are no ridges on prodorsum, but instead there is a characteristic central knot (Fig. 9)
The notogaster of adults can have a pair of lateral wing-like extensions called pteromorphs (Fig. 5). Sometimes the notogaster has shoulder projections (Fig. 10). Other features easy to observe are notogastral setae of different number and length, lyrifissures/cupules (e.g. ia, im), opisthonotal gland opening (Fig.1), porose area (Aa, A1, A2, A3) or sacculi. In some families, the anterior part of notogaster has a photosensitive organ (serving like an eye) called lenticulus (Fig. 11).
An adult has in the ventral aspect (seen from underneath) a genital and an anal plate, and sometimes also an aggenital and an adanal plate. In some families aggenital and adanal plates form one plate, called a ventral plate. The setae present on these plates are called genital, anal, aggenital and adanal setae (Fig. 12).
Habitat
Oribatida can be found in a wide range of habitats: in soil, litter, on plants (including tree canopies), and some species live in fresh waters. In forest soil (to the depth of 10 cm) several hundred thousand individuals representing about one hundred species can be found.
Feeding
All oribatid mites are free-living, and most species are saprophagous (feed on dead organic matter), and they are important for decomposition. Some Oribatida are fungivorous (feeding on fungi), algivorous (feeding on algae), bacteriovorus (feeding on bacteria), omnivorous (feeding on different types of food, including tissue of living plants and carrion), and some species are also predatory, feeding for example on nematodes.
Based on activity of the enzymes cellulase, chitinase and trehalase, species can be classified into seven feeding guilds. Herbivorous grazers that have cellulase activity only, feed on green plants, including algae, and are able to feed on both the cell-contents and the cell-walls. Herbivorous browsers, which do not have the enzymes mentioned above, include most predators, carrion feeders and bacteria feeders. Fungivorous grazers that have chitinase and trehalase activity, feed on fungi and are able to digest both cell-walls and cell contents. Fungivorous browsers, that have trehalase activity only, might be lichenovorous browsers, because trehalose occurs also in lichens. Herbo-fungivorous grazers are able to digest all main food components of green plants and fungi. Opportunistic herbo-fungivores are able to digest cellulose in litter and cell-walls of living green plants and trehalose in fungi. Lastly, omnivores, that have cellulase and chitinase activity, feed on plants and arthropods. Oribatida are prey mainly for other arthropods, like insects and predatory mites from the order Mesostigmata.
Development
Most species reproduce sexually, but about 10% is parthenogenetic (only females are present) and reproduce via thelytoky, which means that females are produced from unfertilized eggs. The female lays several eggs, often in batches, and from each of egg a hexapod (six-legged) larva hatches. The larvae are active for some days, walking and feeding, before they enter a quiescent period. They are then inactive, and they often stay in groups, probably to facilitate better microclimate. After some days the larva molts and an octopod protonymph appears. After some time the protonymph becomes quiescent and molts into a deutonymph, then in the same way the deutonymph molts into a tritonymph, and finally, the tritonymph molts, and the adult appears. The time of development varies, depending on the species and temperature – in warm tropical climate the full development from egg to adult may last one month. In temperate climate this development may last two months, while in the Arctic, five years.
Dispersal
Mites are wingless, so they have rather low dispersal ability. They are mostly transported with wind or water, including seawater, or attached to migrating birds, insects, or other animals.
Ecological importance
Oribatid mites are important for turnover of organic matter in ecosystems, without oribatids decomposition would be much slower. They also improve soil properties and enrich soil with nutrients. They are good bioindicators of the soil health. Only some species are problematic, being intermediate hosts of tapeworms that parasite on livestock and wild animals.
References
Coulson SJ, Hodkinson ID, Block W, Webb NR and Harrison JA (2002). Survival of terrestrial soil-dwelling arthropods on and in seawater: implications for trans-oceanic dispersal. Functional Ecology 16(1), 353-356. doi.org/10.1046/j.1365-2435.2002.00636.x
Lindo Z (2020). Transoceanic dispersal of terrestrial species by debris rafting. Ecography 43(9), 1364-1372. doi.org/10.1111/ecog.05155
Maraun M, Caruso T, Hense J, Lehmitz R, Mumladze L, Murvanidze M, Nae I, Schulz J, Seniczak A and Scheu S (2019). Parthenogenetic vs. sexual reproduction in oribatid mite communities. Ecology and Evolution 9(12), 1-9. doi.org/10.1002/ece3.5303
Norton RA and Behan-Pelletier VM (2009). Oribatida. In: Krantz GW og Walter DE (Eds.), A Manual of Acarology, 3rd Edition. Texas Tech University Press, Lubbock, 421-564.
Pommeresche R and Seniczak A (2018). Jordlevende midd - jordas glemte nyttedyr. NORSØK FAGINFO 7.
Schatz H and Behan-Pelletier VM (2008). Global diversity of oribatids (Oribatida: Acari: Arachnida). Hydrobiologia 595, 323-328. doi.org/10.1007/s10750-007-9027-z
Schatz H, Behan-Pelletier VM, OConnor BM and Norton RA (2011). Suborder Oribatida van der Hammen, 1968. In: Zhang Z-Q (Ed.), Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa 3148(1), 141-148.
Siepel H and de Ruiter-Dijkman EM (1993). Feeding guilds of oribatid mites based on their carbohydrase activities. Soil Biology and Biochemistry 25(11), 1491-1497.
Solhøy T (2001). Oribatid mites. In: Smol JP, Birks JB og Last WM (Eds.), Tracking environmental change using lake sediments, Vol. 4. Zoological Indicators. Kluwer Academic Publishers, Dordrecht, The Netherlands, 81-104.
Walter DE and Proctor HC (2013). Mites: Ecology, Evolution & Behaviour. Springer Dordrecht Heidelberg New York London. doi.org/10.1007/978-94-007-7164-2.
Subías LS (2004). Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes, Oribatida) del mundo (excepto fósiles). Graellsia 60 (número extraordinario), 3-305.
Søvik G (2004). The biology and life history of arctic populations of the littoral mite Ameronothrus lineatus (Acari, Oribatida). Experimental and Applied Acarology 34(1), 3-20.
Thunes KH, Skartveit J, Gjerde I, Starý J, Solhøy T, Fjellberg A, Kobro S, Nakahara S, zur Strassen R, Vierbergen G, Szadziewski R, Hagan DV, Grogan Jr WL, Jonassen T, Aakra K, Anonby J, Greve L, Aukema B, Heller K, Michelsen V.,Haenni J-P, Emeljanov AF, Douwes P, Berggren K, Franzen J, Disney RHL, Prescher S, Johanson KA, Mamaev B, Podenas S, Andersen S, Gaimari SD, Nartshuk E, Søli GEE, Papp L, Midtgaard F, Andersen A, von Tschirnhaus M, Bächli G, Olsen KM, Olsvik H, Földvári M, Raastad JE, Hansen LO and Djursvoll P (2004). The arthropod community of Scots pine (Pinus sylvestris L.) canopies in Norway. Entomologica Fennica 15, 65–90.
Vanschoenwinkel B, Gielen S, Seaman M and Brendonck L (2008). Any way the wind blows - frequent wind dispersal drives species sorting in ephemeral aquatic communities. Oikos 117,125-134.
Weigmann G (2006). Hornmilben (Oribatida). Die Tierwelt Deutschlands. 520 pp. Vol. 76, Goecke and Evers, Keltern.
Siden siteres som:
Seniczak A (2020). Oribatid mites (Hornmidd). www.artsdatabanken.no/Pages/299644. Nedlastet <dag/måned/år>